Cannabis Extraction Methods | Think Higher | Montana MMJ
Enails Vs. Torch: What’s better? | Think Higher Caregiving
February 5, 2021
What Experts Are Saying
December 6, 2021Shatter. Budder. Crumble. Rosin. Understanding the difference between each product is a common query among Montana medical marijuana patients. Aren’t they all the same?
The world of cannabis extracts is vast and fascinating, and it all begins with the many cannabis extraction methods used to create concentrates.

Credit: https://precisionextraction.com
Each concentrate is influenced by a variety of factors during and after the extraction process. Here’s how various cannabis extraction methods influence the final product and what that means for you.
Why are there so many different types of cannabis extracts?
Cannabis extracts come in many different shapes, sizes, and consistencies. This variation is largely based on the extraction process to which the source plant was subjected.

Factors influencing the final product include the solvent used to extract the compounds, the temperature at which the extract was processed, and the methods applied following extraction to alter the final product.
Cannabis extraction methods may be a precise science, but they’re also an art. The final product can be influenced by the extractor’s own flourishes such as the use of a specific solvent, like butane, or for a specific tech such as crystallization.
For example, budder, a fluffy, airy type of wax, is created by applying a whipping tech to the extract at the end of the process. The whipping introduces pockets of air which, when the extract cools, results in a pumice-like construct. Similarly, pouring the extract on a flat tray and allowing it to solidify results in the glass-like structure of shatter.
The different types of extracts represent both creativity and utility. Extractors have experimented with new ways to create different types of extracts by introducing novel techniques into the process.
Additionally, certain types of extracts lend themselves to different uses. For example, shatter is a solid extract that can be easily broken down into small and manageable pieces, reducing the mess often associated with more viscous, sticky extracts.

No matter how you slice it, the result is expanded patient choice, with many options for you to choose from that best fit your lifestyle and needs.
The role of solvent in cannabis extraction methods
The solvent an extractor uses can influence the process. Some solvents are excellent for terpene preservation, for example, while others are more effective at pulling cannabinoids.
Solvents are first chilled, to ensure they are liquid and ran through the biomass (the cannabis material) to soak the material. When the non-polar solvent becomes into contact with the cannabis, the solvent pulls the non-polar cannabinoids, the final result is a liquid solvent full of cannabinoids, terpenes, fats, lipids, etc.
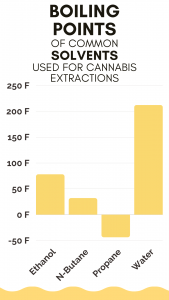
Then the liquid is brought to the solvents boiling temp. The boiling temp of the solvent is normally much lower than the boiling temp of cannabinoids. What is left once the solvent has all boiled off is the hash oil.
Solvents that require higher temperatures for extraction are likely to result in the loss of terpenes more desirable compounds like cannabinoids and terpenes, which is why the solvents with lower boiling points – like the hydrocarbons butane and propane – tend to be popular among extractors.
Water, carbon dioxide, and ethanol are other common solvents used in various cannabis extraction methods, however, those tend to contain a higher amount of fats or lipids and fewer terpenes. One reason they can be more fatty and less terpy can be related to the boiling temp of the solvent used.
What is solvent polarity and how does it influence extraction?
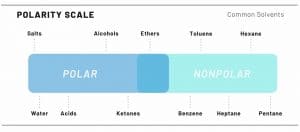
In chemistry, the term polarity describes the separation of electrical charges in a molecule; both solvents and compounds found in cannabis can be varying degrees of polar or non-polar.
In the case of polarity, like attracts like; a non-polar solvent will pull non-polar compounds, while a more polar solvent will pull more polar compounds.

Credit: https://www.abx.org/
As a result, the polarity of a solvent influences which plant compounds are pulled out into an extract. More polar solvents will pull more polar compounds, while less polar solvents pull less polar compounds.
For example, chlorophyll is polar, so using a more polar solvent – such as water – might result in a less clear extract than a less polar solvent, like butane.
While cannabinoids are all non-polar, some terpenes are polar, so the choice of solvent also strongly influences the terpene content of the final product. From least non-polar to most, common solvents include water, ethanol, CO2, butane, and propane.
Depending on which terpenes an extractor wants to pull, and how many, they might select one solvent over another.
Additionally, terpenes have a low boiling point. When the boiling point of the solvent is high, the final extract tends to have fewer terpenes.
That’s because the terpenes are also boiled off with the unwanted solvent. Very little can change this besides the solvent used during the extraction.
Solvent vs. solventless extracts

Solventless extracts are typically produced using heat and pressure alone. Although the term solventless, a solvent is used, and that solvent is water. “Water is called the “universal solvent” because it is capable of dissolving more substances than any other liquid.”
Many people overlook water as being a solvent, but chemistry deems it exactly as that, a solvent.
Rosin is a good example of a solventless extract that is created by high pressures and heat. As a result, a flavorful product is produced that retains the effects of a complex and varied terpene and cannabinoid profile.
Adversely, this product tends to be of a higher fat and lipid content. The process never includes very cold temperatures to filter/process the fats out of the final product therefore they end up in the final product, every time.
Types of Cannabis Extractions
One of the factors which align with the type of solvent used is the type of cannabis extraction that takes place. Extraction methods share similar steps, however, the end product can vary massively.
The type of extraction methods vary in the time it takes to produce the final product and also heavily influence characteristics from color, terpene content, fat content, and even cannabinoid content.
Closed-Loop Extraction: Closed-loop systems are most commonly used with Butane or Propane as the solvent. Closed-Loop systems are used in order to safely recover the solvent after it’s been run through the cannabis biomass.

Credit: https://precisionextraction.com

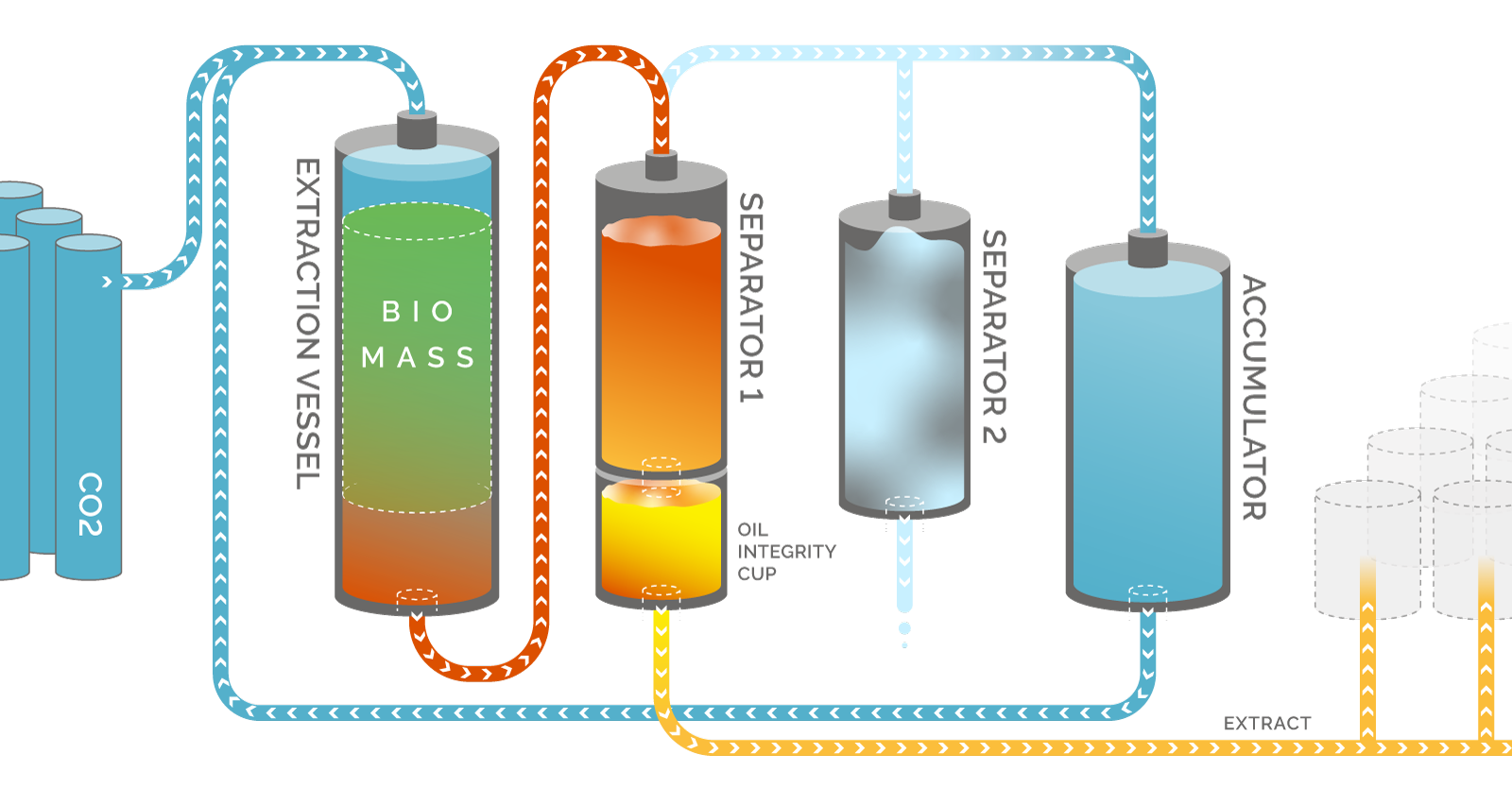
Biomass is first placed into a column. The solvent, which is within a different column, or tank, is then pushed as a liquid, via Nitrogen or by creating a thermodynamic environment, through the column containing the cannabis biomass.
After the solvent runs through the cannabis, it ends within a recovery column. This recovery column is heated in order to evaporate the solvent, leaving behind a rich cannabis oil. The cannabis oil is poured out of the column and then purge within a vacuum oven to remove residual solvent, safe for consumption.
Butane & Propane extractions using a closed-loop system allow a wide range of different concentrates to be produced in a safe & cost-effective manner and why CLS’s are one of the most common methods when it comes to producing concentrates.
Super Critical CO2: Extraction methods using CO2 is another method widely used extraction method. High pressures and ranges of temperature changes are used in order to run the CO2 through the cannabis biomass.
Credit: www.edenlabs.com
When a molecule is in a supercritical state, it has properties of both liquid and gas. CO2 extractions are commonly run within CO2’s supercritical state of both gas and liquid, allowing for maximum contact of the CO2 to cannabis biomass.
CO2 extractions are largely hands-off, however, can be time-consuming, as the CO2 is continuously cycled through the material to maximize the final yield.
The different pressures CO2 extractions undergo widely vary the final product. Specific pressures can more readily concentrate terpenes, while other pressures can provide more cannabinoid content. CO2 extractions can produce some of the cleanest and purest cannabis terpenes when compared to other extraction processes.
Credit: www.edenlabs.com
Concentrates produced by CO2 extractions normally have to undergo winterization as the final product tends to have a higher content of fats and lipids when compared to other extraction methods.
Ethanol Extraction: Ethanol extractions are much more straightforward when compared to other extractions. Cannabis is first put in a vessel, usually a stainless container, similar to a washing machine. Ethanol is then added to soak the cannabis. After stirring and agitation, the cannabis is removed and the ethanol is then evaporated.

Credit: www.edenlabs.com
A large challenge using ethanol is presented by ethanols polarity. Due to the non-polar and polar properties of ethanol, the final product contains unwanted polar molecules such as chlorophyll. Chlorophyll makes the end product more bitter with an undesirable color.
Ethanol extractions are beneficial on large scales, due to the cost when compared to other solvents.
Solvent-Free Extractions: Solvent-free extractions are a large group of products, however, all have one common trait; all were extracted via water, heat, or pressure, or a combo of the three.
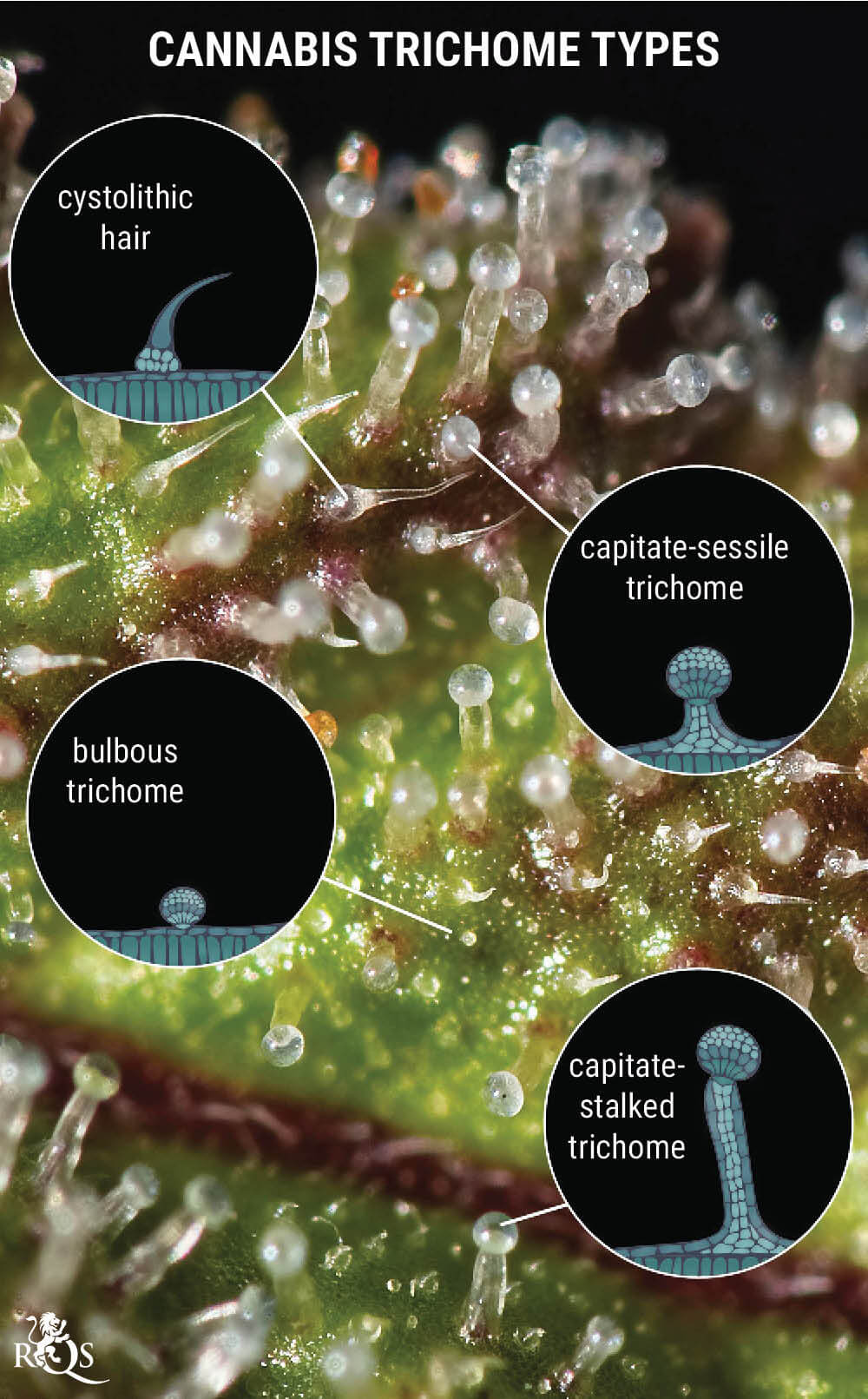
Credit: https://www.royalqueenseeds.com/
The science is quite simple. During solvent-free extractions, trichomes, crystalline formations forming part of the structure containing the terpenes and cannabinoids, are first removed from the cannabis flowers.
The separation of trichomes can be done in multiple ways. One common method is by performing a cold water extraction to separate the trichomes. Cold water is washed over the cannabis in bags that have different-sized screens. These screens separate the trichomes and other molecules, based on their size, or micron.
Trichome heads, the most potent area, are extremely tiny when compared to other components such as trichome stalks.
After the trichomes have been extracted they can be manipulated in a number of ways to produce a number of final products. Old school hash is produced by working these trichomes together by hand. Working them mechanically together producing a dark, sticky, potent substance.

Credit: https://www.trimleaf.com/
Rosin also incorporates the trichomes by using heat and pressure. The trichomes are placed together in a bag, that is then placed in a press that has heated plates. Using high amounts of pressure, the heat plates press down on the trichome-filled bag that essential “juices” the trichomes.
How cannabis extraction methods influence the final product
After solvents (or solventless methods) have been used to extract the compounds from the source plant, there are several techniques extractors can employ to create the final product. Some of these techniques include:
Purging: Once an extract is pulled from a plant, the extract may have some low-boiling solvents left behind. Purging removes these unwanted, excess solvents from the extract. This is typically done by using a vacuum oven and a vacuum pump.

The oven allows the extractor to heat while the pump allows the extractor to pull a negative vacuum on the product to pull more unwanted solvent. The equipment used to do this and the temperatures at which purging is done can influence the look, feel, and cannabinoid and terpene content of the final product.
CRC: Another technique, known as Color Remediation Cartridge (CRC), influences the appearance of the final product through silica, carbon, clays, and other mediums.

Credit: lunatechequipment.com
These mediums pull different pigments and other impurities from the plant & wax, resulting in a unique, colorful appearance in the final product. For example, using bentonite clay during the extraction process tends to pull red and green pigments from the plant, while different mediums instead pull yellows and golds.

Credit: Future4200.com
Post-extraction manipulation: Once an extract has been pulled from a plant, extractors can apply several treatment types to create the final product.

Whipping an extract, for example, results in a light and fluffy budder. However, it also causes more terpenes to degrade when exposed to heat and air, so budder trades terpenes for a more aesthetically pleasing and manageable final product. Shatter, on the other hand, preserves more terpenes when compared to budder.
Extractors can also use mechanical devices, like centrifuges, to purify products like THCA diamonds, which are largely devoid of terpenes and helps result in a product that is up to 99% pure THCa.
What are the available cannabis extracts?
Extracts are products derived from cannabis flower that contain concentrated amounts of cannabinoids, like THC and CBD, and terpenes, the flavorful and aromatic volatile organic compounds known for giving cannabis strains unique flavors and enhancing their effects.
Concentrated extracts are growing in popularity because they’re much more potent than flower. In fact, extracts remain one of the fastest-growing sectors of the cannabis industry. Here’s a look at some of the most common types of cannabis extracts you might encounter at your local dispensary.

- Oil: Cannabis oil is a highly viscous extract, typically produced using carbon dioxide as a solvent. They are most commonly found in vaporizer cartridges. The use of CO2 as a solvent tends to result in the preservation of more terpenes for a more flavorful product; however, the process takes much longer than other solvents and can result in damage to the cell walls of compounds in the extract.

- Wax: Wax is an extract named for its appearance and consistency, typically produced using butane as a solvent. Wax can be very sticky and messy to handle, but wax comes in many shapes and sizes, some of which aren’t sticky at all. Extractors often whip wax after its creation, resulting in a light, fluffy type of wax called budder. Other forms of wax include badder and crumble, which are similarly aptly named.

- Shatter: The extract is then poured thin and flat on a tray, where it solidifies into a glass-like structure that can easily be broken into smaller pieces for consumption. Multiple different solvents can be used to produce a shatter, however, Butane shatter tends to be the best of both worlds when it comes to THC percentage and terp retention. At Think Higher, we use butane to produce shatter.

- Sugar: Sugar is a special type of cannabis wax that is dry and grainy, making it much easier to handle than many other varieties of butane or ethanol extracts. Sugar is known for its high potency and crystalline consistency, making it easy to handle with much less mess for dabbing. Sugar is a perfect mix between THCa and Terps.

- Rosin: Rosin is a solventless extract made only with the use of low-temperature heat and pressure. This process preserves flavor, as this method preserves terpenes due to the lower temperatures used. Rosin is produced in a similar major to “juicing” apples for apple juice, however, in rosin it’s the “juicing” of the trichome glands.
- Live Resin: Live resin is made by flash freezing freshly harvested cannabis prior to the conventional drying and curing phases, then extracting from the flower at subcritical temperatures. This process is designed to retain a high level of terpenes while minimizing lipids and other fatty content. Live resin is a general category that can be broken down into various categories within itself. You may see live resin as a sauce, high terpene extract, and diamonds.
- THCa / Diamonds / Crystals: THCa crystalline is the
 purest form and sometimes referred to as THC crystals or THC diamonds. Using the term THC for these concentrates is actually incorrect, THC doesn’t crystallize, unlike THCa. THCa is refined to the crystal form and although diamonds lack the flavor, due to having no terpenes, diamonds pack a heavy punch. THCa comes in a wide variety of sizes and shapes depending on specific techniques and solvents used.
purest form and sometimes referred to as THC crystals or THC diamonds. Using the term THC for these concentrates is actually incorrect, THC doesn’t crystallize, unlike THCa. THCa is refined to the crystal form and although diamonds lack the flavor, due to having no terpenes, diamonds pack a heavy punch. THCa comes in a wide variety of sizes and shapes depending on specific techniques and solvents used.
- Sauce: Sauces are normally a liquid and sticky wax
 incorporate with THCa diamonds. The tasty combo of both THCa crystals and terpenes provides the best of both flavor and effect. Sauces pack a ton of flavor but also allow the consumer to take a wider spectrum of hits; more terps and less THCa will provide a more flavorful hit, where grabbing just a THCa diamond will provide a heavy psychoactive feel.
incorporate with THCa diamonds. The tasty combo of both THCa crystals and terpenes provides the best of both flavor and effect. Sauces pack a ton of flavor but also allow the consumer to take a wider spectrum of hits; more terps and less THCa will provide a more flavorful hit, where grabbing just a THCa diamond will provide a heavy psychoactive feel.
- Applesauce: Applesauce is a term used to describe the texture and
 consistency of the recrystallization phase that occurs after separating the initial THCa crash from the mother liquor (terpenes). THC & THCa are still present within this mother liquid, although in much smaller percentages when compared to the initial crash. Once separated the mother liquid will begin to recrystallize under certain conditions. Applesauce can be compared to that of small sugar crystallizes that have terpenes incorporated within the matrix. Applesauce consistency concentrates, similarly to sauces, contain quality flavor on top of a quality high. Another term widely used for applesauce is sugar crystals.
consistency of the recrystallization phase that occurs after separating the initial THCa crash from the mother liquor (terpenes). THC & THCa are still present within this mother liquid, although in much smaller percentages when compared to the initial crash. Once separated the mother liquid will begin to recrystallize under certain conditions. Applesauce can be compared to that of small sugar crystallizes that have terpenes incorporated within the matrix. Applesauce consistency concentrates, similarly to sauces, contain quality flavor on top of a quality high. Another term widely used for applesauce is sugar crystals.
 High Terpene Extract / High Terpene Full Spectrum Extract: High terpene full-spectrum extracts are unique concentrates that normally contain roughly 50% THCa and anywhere from 10%-40% terpenes. The variance is based on the composition of the starting plant material and the extraction method performed. HTE’s and HTFSE’s can be used in many different ways from dabbing directly to adding to carts. HTE’s are the perfect way to add to other products such as flavoring pure THCa diamonds or topping joints and bowls.
High Terpene Extract / High Terpene Full Spectrum Extract: High terpene full-spectrum extracts are unique concentrates that normally contain roughly 50% THCa and anywhere from 10%-40% terpenes. The variance is based on the composition of the starting plant material and the extraction method performed. HTE’s and HTFSE’s can be used in many different ways from dabbing directly to adding to carts. HTE’s are the perfect way to add to other products such as flavoring pure THCa diamonds or topping joints and bowls.
It is important to note that there is a lack of standardized naming conventions in the cannabis industry. This means that product names may vary from company to company.
There is no clear right or wrong, but the variability can be confusing. While the list above describes extracts by some of their most common names, you might encounter some products made with the same cannabis extraction methods, but with different labels.
Understanding cannabis extraction methods guarantees quality
Cannabis extraction methods and processes are complex and in-depth, sometimes making it inaccessible to many consumers.
Knowing more about how your extracts are produced can help you not only guarantee you are purchasing a quality product, it can help you to make a more educated selection of a product that will best fit your needs.

If you have any questions at all about our extraction processes or how they influence your final product, contact Think Higher Caregiving. We can explain these complex processes to you in plain language so that you can be sure you go home with the cannabis extract that will help you feel better.

